
 |
|||
 |

|

|
 |
|
Confocal-Rheology of Active Microtubule Networks
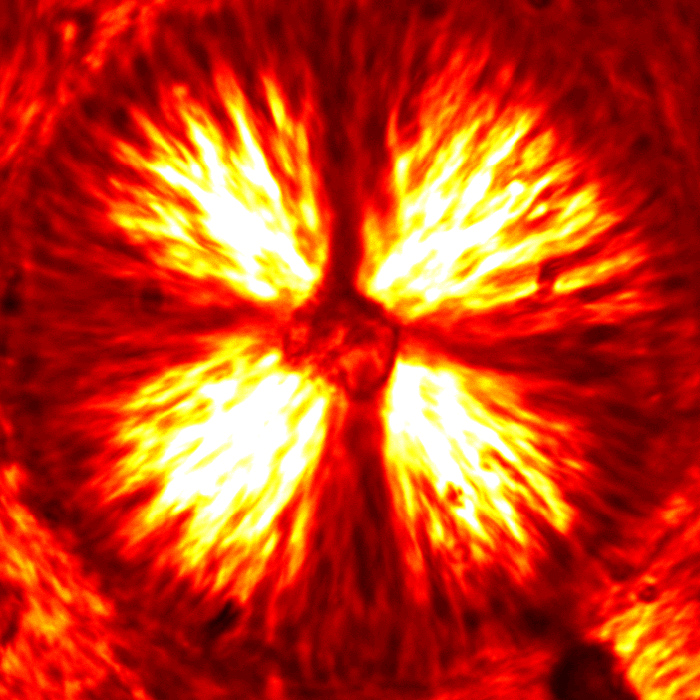
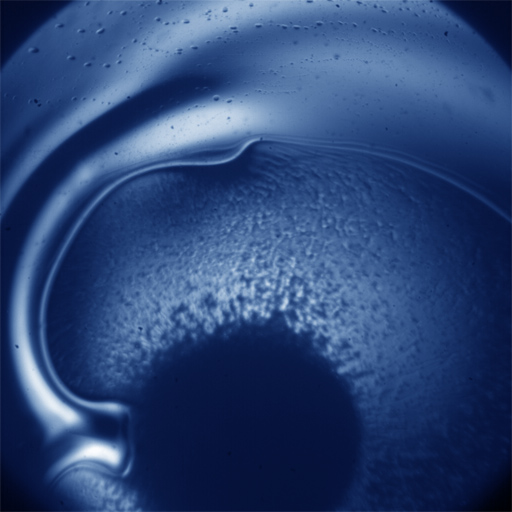
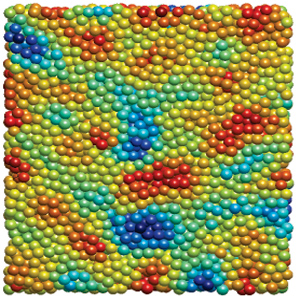
This case study is focused on the rheological characterization of simplified viscoelastic active gels, standing out from the concept of conventional well-defined gel structures (i.e., passive gels, either temporary or permanently cross-linked networks). The main ingredient of such active systems is stabilized microtubule (MT) filaments, one of the constituents of cytoskeleton that has the fundamental role to give cells structure and shape. As filament, MTs can be referred as stiff polymer chains. They are made of many single subunits called tubulin linked together both at their ends and along their side giving the typical tubular structure (~25 nm diameter), as depicted in Figure 1a. By adding a depleting agent (such as polyethylenglycol (PEG) polymer) and clusters of molecular motor kinesins, MTs start bundling due to the well-known depletion mechanism, and link to each other (Figure 1b). Kinesins are remarkable biological nanomachines that are capable of moving along a microtubule track in the presence of energy carriers, such as adenosine triphosphate (ATP). They convert chemical energy from ATP hydrolysis into a sustained movement (Figure 2). As kinesin clusters simultaneously bind and move along multiple filaments, MT bundles can form a highly dynamical and 3D percolating network that undergoes continuous rearrangements. Figure 3 shows the 3D reconstruction of a percolated active MT network at microscopic scale. It is apparent that the dynamics of such active systems is driven by repeating cascades of bundles undergoing internally motor-driven polarity sorting, extension, buckling, fracturing and recombination (Figure 3). Such microscopic dynamics drives large-scale streaming and mixing flows that span the entire gel and appear chaotic, lacking any directionality, far from the equilibrium state. 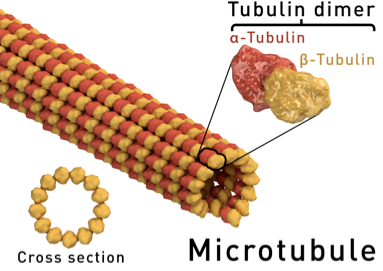 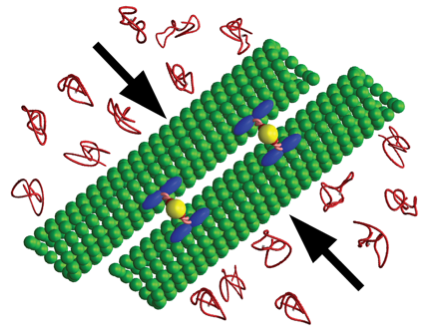
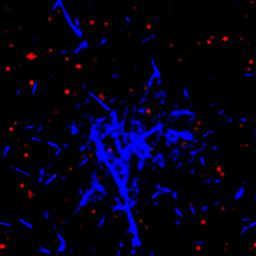
In comparison to extensively studied passive gels, viscoelastic properties of active gels remain poorly understood. For example, it is not immediately obvious if the MT-based active gels exhibit properties that are more like those of a conventional liquid or a solid. In this work we quantify the viscoelasticity of non-equilibrium MT-based active gels and determine how it depends on the relevant molecular parameters, which are easily tunable in our highly simplified model system. Until recently, the interpretation of rheological measurements was mostly dependent on microscopic theoretical models, largely due to the limited experimental data that relate fluid flow to the dynamics of the microscopic or mesoscopic constituents. To overcome these limitations, we combine time-resolved 3D confocal microscopy to stress-strain controlled bulk rheology measurements. In particular, using a combination of confocal imaging and quantitative image analysis, we are able to reconstruct 3D topology of the active gel and quantify its temporal recombination dynamics (Figure 3). Such data is essential for developing theoretical models of active gels, yet it is currently missing. Preliminary observations suggest an intriguing possibility that active MT gels in their simplest state mimic certain mechanical properties of the intracellular environment. Furthermore, varying the ATP concentration has a significative impact of MT activity on the time scale response (Figure 4). The outstanding challenge is to correlate all the possible active dynamics to their characteristic times and understand the nature behind this spectacular phenomenon.
|