
 |
|||
 |

|

|
 |
|
The glass
transition in P-NIPAM microgels
A colloid is a mixture made up of particles large enough to be
chemically distinct, although they are not so large that they
settle. First defined by Thomas Graham in the nineteenth
century, the study colloidal systems has emerged a science that lets us
understand many useful and interesting behaviors. One of
these behaviors occurs when the packing fraction is
increased. At a certain point, suspended particles pack so
closely that each particle is effectively caged. The
suspended material is no longer free to move throughout the
system. The system is a glass.
We are examining glassy colloidal suspensions; particularly, what happens as the dispersions becomes glassy. There are many ways to concentrate a colloidal suspension to create a glassy system. Most of these, such as centrifugation or dialysis, involve physical manipulation of the system, making it difficult to understand how the colloid enters the glassy state. Instead, we are looking at glassy systems made from n-isopropyl acrylamide (NIPA), a thermoresponsive material that swells as temperature decreases. With colloids made from these materials, we can control volume fraction simply by changing temperature. All this can be done while visualizing the material under the microscope. We have developed stable (to flocculation), fluorescently labeled, monodisperse NIPA particles that we can readily visualize in a confocal microscope. We are just beginning to understand the behavior of these NIPA particles with volume fractions at or near the glass transition. An interesting complication to our study is that we have found that the size of these NIPA particles is sensitive to temperature as well as volume fraction—this sensitivity is really a response to increased osmotic pressure which is intimately connected to volume fraction. 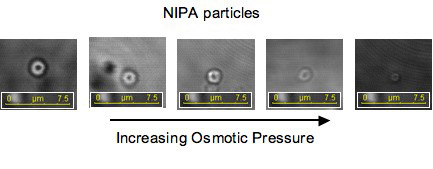 The relationship between volume fraction and particle size poses some difficulties, but also makes our study much richer. We expect that smaller particles are ‘harder’ than the larger ones of the same composition. Combining our thermal control of size with our understanding of how osmotic pressure affects size; we have easy access into what happens as colloids become glassy, as well as how particle hardness affects the dynamics. For more information on this project please contact Dan Blair |