
 |
|||
 |

|

|
 |
|
Rheology of
Compressed Emulsions
An
interesting property of sand is that it can easily be poured out of a
bucket, yet a sand pile can support a great deal of weight.
The grains
behave much like a liquid at low number density and high shear, but
when the grains are packed tightly and the shear is removed, the system
undergoes the so-called jamming transition and displays solid-like
behavior. While a wide range of disordered systems (including
foams, gels,
and emulsions) exhibit similar phenomena, whether they can all be
accurately described by a universal theory of jamming remains an open
question.
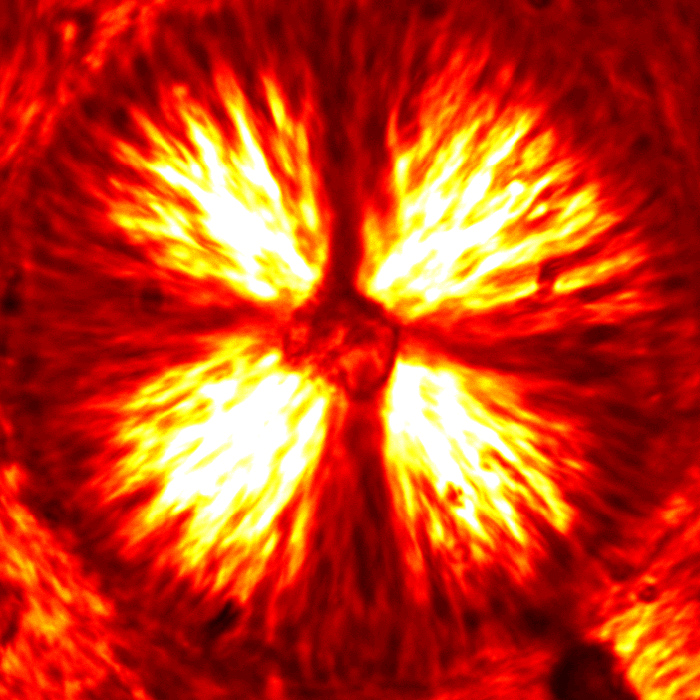
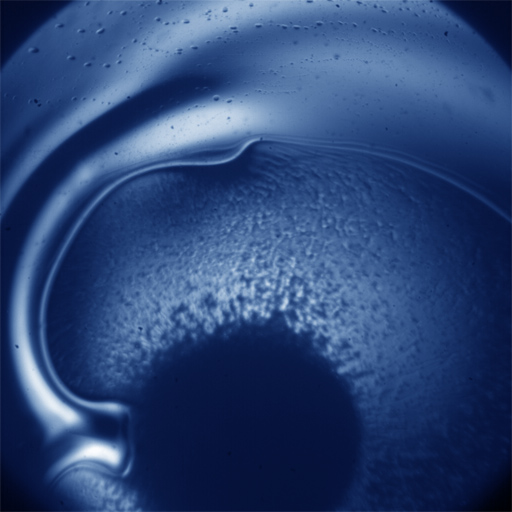
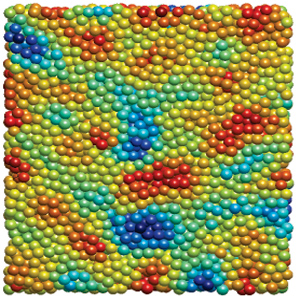
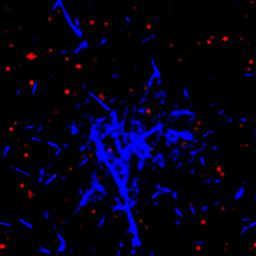
We are studying an oil-in-water emulsion, which has several useful properties for investigating jamming. The size distribution of oil drops can be set relatively easily; we typically use 5 um drops with a polydispersity of around 20%, which is high enough to prevent crystallization. By adding glycerol to the water, we can index match both phases of the emulsion, making it optically clear. A confocal microscope can then see deep within the sample and create a three-dimensional image. Either phase can be fluorescently labeled, but when Nile Red is used to dye the oil, contact patches can be directly visualized, as these regions strongly emit at higher wavelengths. The force between two drops can then be calculated from the patch size and the surface tension of the oil/water interface. 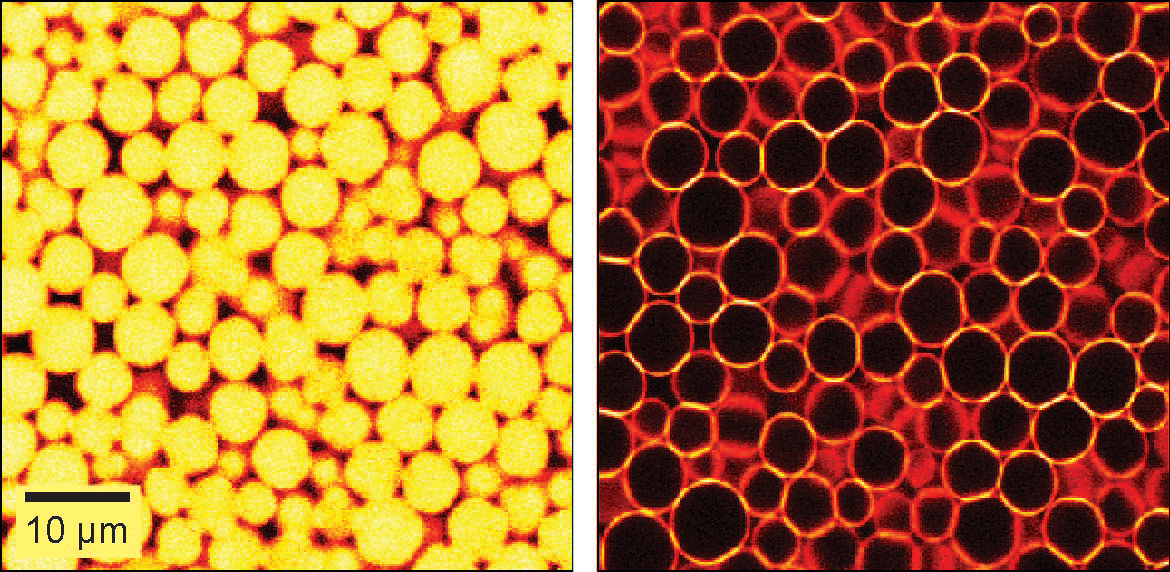 An emulsion can be characterized on two different length scales. The bulk viscoelastic properties can be measured with a rheometer, while the the confocal microscope provides information at the droplet level. At low applied shear strain, the storage modulus G' is roughly constant and larger than the loss modulus G'', as one would expect from a good solid. Microscopically, the applied shear force causes a reversible deformation of individual drops, corresponding to energy storage. As the strain increases, the emulsion yields, and G'' becomes larger than G'. Here, the shear is large enough to cause drops to slide past each other, resulting in dissipation. 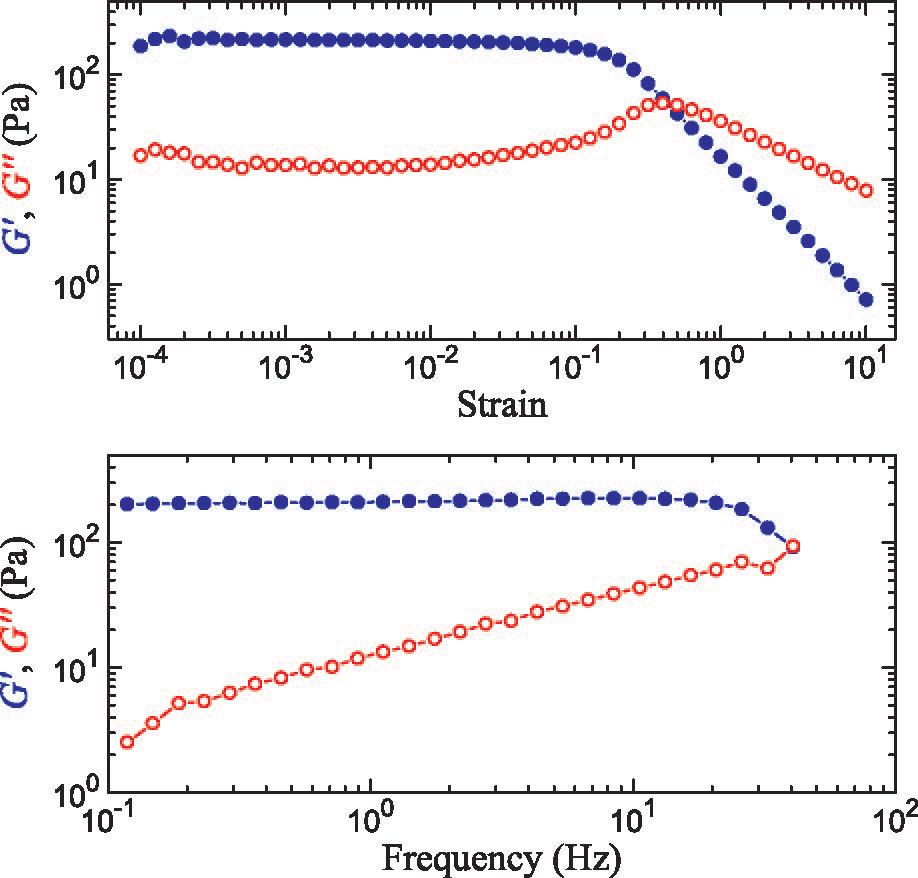 By using the confocal rheometer in our lab, we are trying to reconcile the microscopic and macroscopic pictures of the emulsion. In particular, the distribution of contact forces throughout a sample under strain is of great interest. Unlike in an ordered crystal, the shear force is not evenly distributed among the constituent particles, but instead largely conveyed along chains that span the sample. It is thought that the broad distribution of forces follows a universal form and changes qualitatively as the systems leaves the jammed state. In addition, slippage at the boundaries, the distribution of neighbors, and the homogeneity of the dynamics can be directly measured, providing some insight into the nature of the jammed state. For more information on this project please contact Dan Blair. |